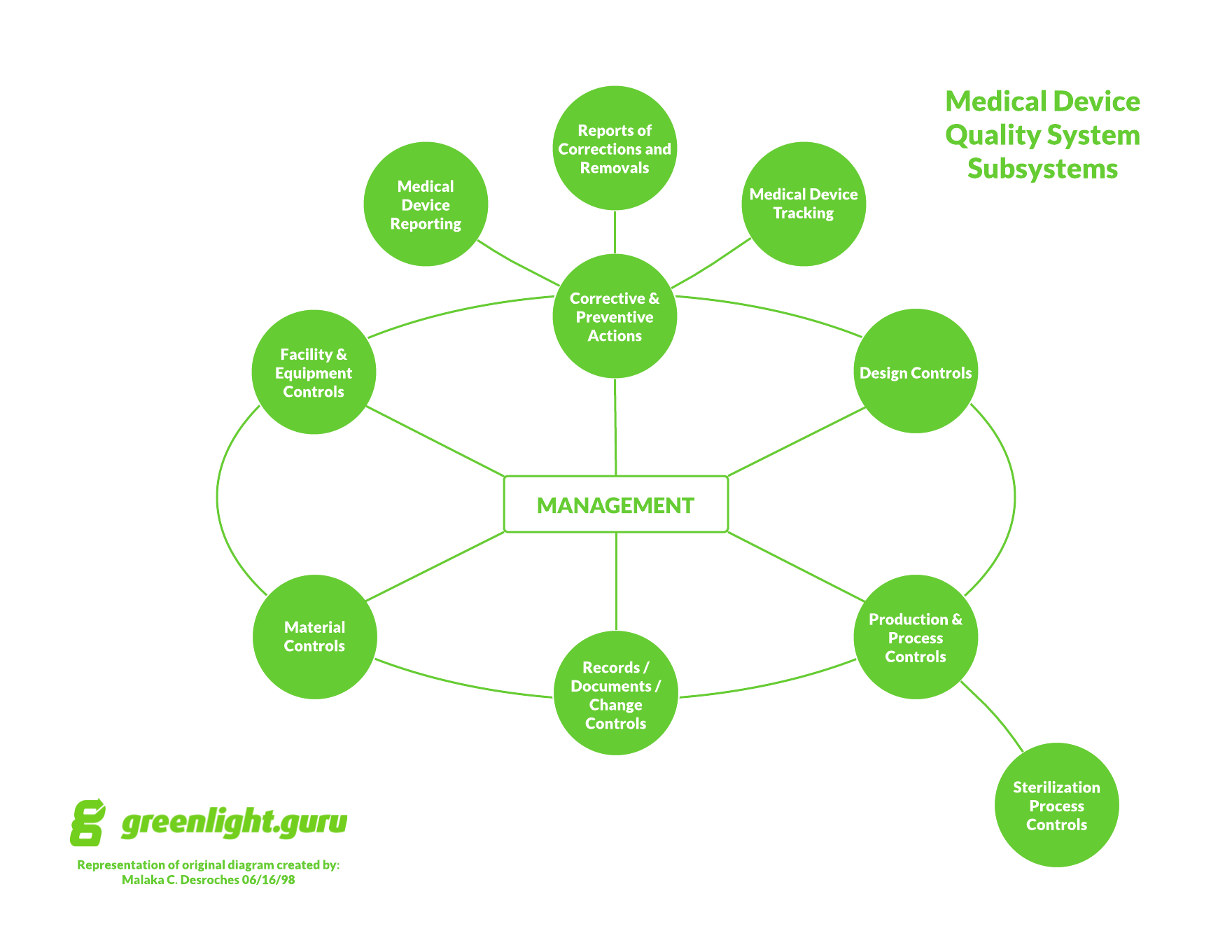
I love a scavenger hunt as much as the next person.
However, when it comes to medical device documents and records, your system and approach should not be the makings of a scavenger hunt.
Whether you have been through this before or if this is the first time, I want you to take a few moments to consider the importance of the documentation of your medical device company.